Gas Constant Air in English Units
Where P Pressure bar atmosphere Pa V Gaseous volume m 3 cm 3 n number of gaseous moles dimensionless R Universal gas constant JmolK litatmmolK T Temperature of the gas K 0 C R is also known by alternative names such as Ideal gas constant molar gas constant or simply R gas constant. The air density can be calculated with a transformation of the ideal gas law 5 to.
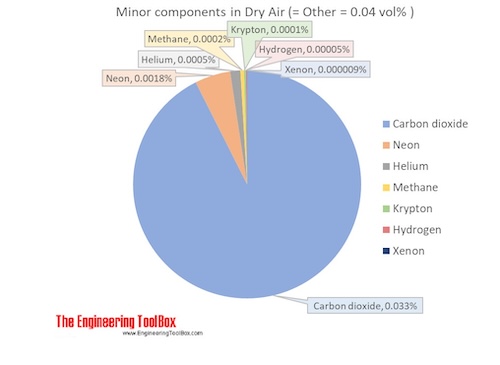
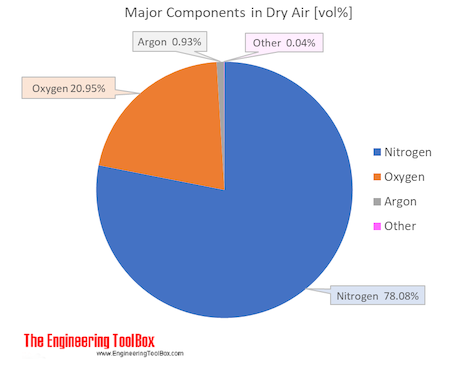
Air Composition And Molecular Weight
Mmixture ΣxiMi x1M1 xnMn 4 where xi mole fractions of each gasMi the molar mass of each gas The Universal Gas Constant - Ru - in alternative Units atmcm3molK.

. 0 F 45967R 0C27315K 1K18R Pressure. The ideal gas law in terms of R u is. Temperature in degrees Fahrenheit F.
General gas constant is R 831 J K-1 mol-1. PROPERTY TABLES AND UNITS 3 TABLE A-2UNIVERSAL GAS CONSTANT FOR DIFFERENT UNITS Pressure Unit Volume Unit Temperature Unit Mass mole Unit Gas Constant R psia ft3 R lbm 107315 psia cm3 R lbm 303880 psia cm3 Rg66994 bar ft3 R lbm 073991 atm ft3 R lbm 073023 atm cm3 Rg45586 Pa m3 Kkg83143 Pa m3 Kg83143 kPa m3 Kkg83143 kPa cm3. Universal gas constant and ideal gas law.
The specific gas constant for dry air is 287058 JkgK in SI units and 5335 ftlbflbR in United States customary and Imperial units a quantity that may vary slightly depending on the molecular composition of air at a particular location. The gas constant is inversely used in diverse disciplines. Heat of vaporization L v.
Temperature R or K. The same regardless of the gas being considered. Universal gas constant and ideal gas law The universal gas constant Ru is as its name implies universal ie the same regardless of the gas being.
If you are using kg mol as the unit for quantity of matter then multiply the value given below by 1000. The value of the gas constant in SI unit is 8314 J mol 1 K 1. 947 PROPERTY TABLES AND CHARTS ENGLISH UNITS Table A1E Molar mass gas constant and critical-point properties 948 Table A2E Ideal-gas specific heats of various common gases 949 Table A3E Properties of common liquids solids and foods 952 Table A4E Saturated waterTemperature table 954 Table A5E Saturated waterPressure table 956.
Ft 2 or m 2. I have ran into a problem in converting from imperial to SI units as follows. The quantity of heat required to convert a unit of liquid at a specific temperature into its vapor at the same temperatureThe value of this quantity is usually given at the normal boiling point of the liquid and is measured in kJkg or.
Temperature in degrees Celsius C. Gas Constant Values based on Pressure and Volume Units. Values of R Gas Constant Value Units VPT 1n1 8314 462175 J K1 mol1 5189 1019 eV K1 mol1 0082 057 4614 L atm K1 mol1 1985 877534 cal K1 mol1 1985 877534 103 kcal K1 mol1 8314 462175 107 erg K1 mol1 8314 462175 L kPa K1 mol1.
Some say the symbol for the gas constant is named in honour of. The molecular weight of elemental atomic nitrogen for example in English units is Mnitrogen 140067 lbmlbmol and the molecular weight of air is air lbm 2897 lbmol M. Hi Im creating an inventory of conversion factors for various reference gases for air monitoring.
Universal gas constant R u 831451 J mol K 198589 Btu mol R. The ideal gas constant for air is often given in imperial units as texR 1716 fracftlbfslugR tex where tex 1 ftlbf 135610-3 kJ tex tex 1 slug 1459 kg tex tex R frac95K tex Thus making these substitutions gives. This table is based on g moles as the unit for quantity of matter and Kelvin as the unit for temperature.
Air gas constant is RairR289702869 Jg K2869 Jkg K Wiki User. Gas constant R 8314459848 Jmol 1 K 1. Gas Constant R in English Units.
The digits inside the parentheses are the uncertainty in the measurement of gas constant value. To standardize molesvol Ive noticed that many air districts start with R in the familiar units 082057 latm kmol then do a series of conversions to reach english units. The origin of the symbol R for the ideal gas constant is still obscure.
Hence it is expressed in many units. Temperature in degrees Rankine R. R 10731573ft³psiaRlbmol 073024026ft³atmRlbmol 820573383cm³atmKgmol 00831446LbarKgmol 83144598m³PaKgmol 00831446m³barKkgmol 19858746BtuRlbmol 19858746calKgmol 83144598JKgmol Temperature.
ρ p R T 7 ρ 50 lbin 2 147 lbin 2 144 in 2 ft 2 1716 ftlbslug. Heat transfer rate W m 2 8806 1 0 5 Btu ft 2 s. Including the value of the molecular weight we can define a particular gas constant R for air.
Specific gas constant for water vapor. The universal gas constant R u is as its name implies universal ie. The atomic weight of elemental nitrogen for example in English units is A rnitrogen 140067 lbmlbmol and the molecular weight of air is.
There is a universal gas constant which relates these variables and the molecular weight of any gas. The dimension of the gas constant is expressed in energy per unit mole per unit temperature. Some gas constant value in different units are listed below-.
The gas constant has the same unit as of entropy and molar heat capacity. FtlbfslugR or m 2 Ks S. Each gas multiplied by the molecular weight of that particular gas.
Ideal-gas specific heats of various common gases a At 80F Gas constant Rc p c v Gas Formula BtulbmR BtulbmR BtulbmR k Air 006855 0240 0171 1400 Argon Ar 004971 01253 00756 1667 Butane C 4H 10 003424 0415 0381 109 Carbon dioxide CO 2 004513 0203 0158 1285 Carbon monoxide CO 007090 0249 0178 1399 Ethane C 2H. O R 70 460 R 00102 slugsft3 The weight of the air is the product of specific weight and the air volume. Specific gas constant for air.
Gas Constant In Different Units. FtlbfslugR or m 2 Ks R e. The state of a gas can be changed by external processes and the reaction of the gas can be predicted using the laws of thermodynamics.

No comments for "Gas Constant Air in English Units"
Post a Comment